the number of resonance structures for n is|how to tell resonance structures : Cebu BOi is the bond order of the identified bond in a resonance structure. FCi is the formal charge of the identified atom in a resonance structure. n is the number of resonance . We would like to show you a description here but the site won’t allow us.
0 · the number of resonance structures possible for is are ____
1 · resonating structures of alpha naphthol
2 · resonance structures with triple bonds
3 · nitrogen resonance structure diagram
4 · how to tell resonance structures
5 · how to count resonance structures
6 · how many resonance structures n
7 · draw all significant resonance structures
8 · More
A Bantubet.co.ao é licenciada e regulada pelo Instituto de Supervisão de Jogos de Angola, de acordo com a Licença de jogos on-line Nº B20081200360166656754/ISJ/MF/20 .
the number of resonance structures for n is*******Resonance structures are used when one Lewis structure for a single molecule cannot fully describe the bonding that takes place between neighboring atoms relative to the empirical data for the actual bond lengths between those atoms.
BOi is the bond order of the identified bond in a resonance structure. FCi is the .
Resonance is a way of describing delocalized electrons within certain .When drawing a resonance structure there are three rules that need to be followed .the number of resonance structures for n is how to tell resonance structuresBOi is the bond order of the identified bond in a resonance structure. FCi is the formal charge of the identified atom in a resonance structure. n is the number of resonance .the number of resonance structures for n isSolution. Verified by Toppr. Naphthalen-2-ol acts as an acid and reacts with N aOH to form salt sodium naphthalen-2-olate which is compound N. The number of resonance structures for N is 9. They are as shown in the . Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis . When drawing a resonance structure there are three rules that need to be followed for the structures to be correct: Only electrons move and the nuclei of the atoms never move. Only electrons that can .In cases in which more than one reasonable (plausible) Lewis structure can be drawn for a species, these structures are called resonance structures or resonance contributors. Resonance structures can be .Learn what resonance structures are and how they describe the delocalization of electrons in molecules or ions. See examples of resonance structures for NO2-, NO3-, O3, CO32-, .We call the individual Lewis structures resonance forms. The actual electronic structure of the molecule (the average of the resonance forms) is called a resonance hybrid of .The number of resonance structures for ‘N’ is ___. Q. Greater the number of resonating structures for a given intermediate. Q. The structures for compounds (A) and (B) are .
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or .The bottom left has 7 electrons and 6 – 7= -1. To find the number of valence electrons, refer to the group number at the top of the periodic table. Step 1: Transfer the electrons on the oxygen with the negative charge and turn it into a double bond. Step 2: Break the double bond between the positive oxygen and the neutral oxygen. Each structure is called a resonance structure, and they can be connected by the double-headed resonance arrow. There are total three equivalent resonance structures for CO 32-, and the actual . Anthracene will undergo delocalisation to give four resonance structures. The resonance structure of Anthracene is given below: Therefore, the correct answer is option (C) 4. Note: Anthracene .
Telegram :- https://t.me/chemshikshaofficialsThe number of resonance structures for N isINSTAGRAM :- https://www.instagram.com/chemshiksha/FACEBOOK :- https:.
Resonance structures of NO 3-ion. Lets draw the three resonance structures for the nitrate anion (NO 3-). Lone pairs, charges and bonds of NO 3-ion. When we draw resonance structures, we convert lone pairs to bonds and bonds to lone pairs when it is possible.. In lewis structure of NO 3-ion, there are three lone pairs (in the last shell) in .Resonance (chemistry) In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or forms, [1] also variously known as resonance structures or canonical structures) into a resonance hybrid (or hybrid structure) in valence bond .How to Draw Resonance Structure [4-8] Rules of Resonance Structure. In order to draw the resonance structures, one has to keep the following rules in mind: They must have the same number of electrons. They must be valid Lewis dot structures. There is no change in hybridization between the structures. Only electrons move through the molecules.
When writing resonance structures keep in mind that THEY ALL MUST BE VALID LEWIS FORMULAS. The factors that make up valid Lewis formulas are as follows. 1. Observe the rules of covalent bonding, including common patterns as discussed previously. Make sure to show all single, double, and triple bonds.Match the number of resonance structures to the correct compound. nitric acid, HNO3 ( N is 1. One resonance structures can be bonded to an OH group created. and two O atoms) 2. Two resonance structures can be nitrate ion, NO3 3. Three resonance structures can be created. selenium dioxide, OSeO 4.
Subtract this number from the number of valence electrons for the neutral atom: I: 7 – 8 = –1 Cl: 7 – 7 = 0 . This gives rise to three resonance forms of the carbonate ion. Because we can write three identical resonance structures, we know that the actual arrangement of electrons in the carbonate ion is the average of the three .The number of resonance structures for N is: The structures for compounds (A) and (B) are given. Also, resonance structures I, II, and III for each compound (A) and (B) are given.
All resonance structures must have the same atom connectivity, and only differ in the electron arrangement. (Atoms NEVER move, only electrons move.) All resonance structures have the same number of electrons and net charge. (Formal charges on individual atom could be different, but net charge, that is the sum of all the .
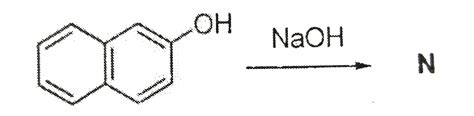
Q. The number of resonance structures for N is: Q. The number of resonance structures for N is ___. Q. The number of resonance structures for ‘N’ is ___. Q. Greater the number of resonating structures for a given intermediate. Q. Write the resonance structures for SO3, N O2 and N O− 3 .The number of resonance structures for N is. The number of resonance structures for N is.
The number of resonance structures for N is: The structures for compounds (A) and (B) are given. Also, resonance structures I, II, and III for each compound (A) and (B) are given.
All resonance structures must have the same atom connectivity, and only differ in the electron arrangement. (Atoms NEVER move, only electrons move.) All resonance structures have the same .Q. The number of resonance structures for N is: Q. The number of resonance structures for N is ___. Q. The number of resonance structures for ‘N’ is ___. Q. Greater the number of resonating . The number of resonance structures for N is. The number of resonance structures for N is.Each structure is called a resonance structure, and they can be connected by the double-headed resonance arrow. There are three equivalent resonance structures for CO32-, and the actual structure of CO32-is a hybrid of the three resonance contributors. Figure 1.3b Three equivalent resonance contributors of carbonate anion. Now that you know the number of valence electrons per element, you need to compute the total valence electrons for the NO_3^"-1" ion. 5 + (3 x 6) = 23 electrons But since the whole molecule has a -1 charge, you need to add this too. So the total number of valence electrons is 24. The next thing to do is draw.Resonance structures of NO 2-. Lets draw the two resonance structures for the nitrite anion NO 2-. Lone pairs, charges and bonds of NO 2-ion. When we draw resonance structures, we convert lone pairs to bonds and bonds to lone pairs if it is possible.. In lewis structure NO 2-ion, there are three lone pairs (in the last shell) in one oxygen atom and .how to tell resonance structuresPhenols behave as weak acids and Naphthalen-2-ol is an phenol; So Naphthalen-2-ol acts as an acid and reacts with N a O H to form salt sodium naphthalen-2-olate which is compound N.; The number of resonance structures for N is . Dinitrogen monooxide, or #"N"_2"O"#, has three resonance structures, out of which one is a major contributor and one is a minor contributor.. The #"N"_2"O"# molecule has a total number of #18# valence electrons - 6 from nitrogen and 6 from each oxygen atom.. The three resonance structures for dinitrogen monoxide are. All the .The three possible resonance structures of NO 3– are illustrated below. If a resonance hybrid of this polyatomic ion is drawn from the set of Lewis structures provided above, the partial charge on each oxygen atom will be equal to - (⅔). The net charge on the central atom remains +1. This resonance hybrid is illustrated below.
Dinitrogen pentoxide is an strong acidic oxide and nitrogen atom is at +5 oxidation state. Here we are going to draw lewis structure and resonance structures of N 2 O 5 molecule.. Drawing the lewis structure of N 2 O 5. skeletal structure of N 2 O 5 molecule is below. We are going to find, how σ bonds, π bonds and lone pairs are located in this .

24.2k views. asked Mar 31, 2018 in Chemistry by shabnam praween (138k points) The number of resonance structures for N is. jee. jee mains. Share It On.
Subscribe to the YouTube Music channel to stay up on the latest news and updates from YouTube Music. Download the YouTube Music app free for Android or iOS.
the number of resonance structures for n is|how to tell resonance structures